The McRae lab is currently funded by a 5-year CPRIT recruitment grant to perform structure-based design of RNA therapeutics.
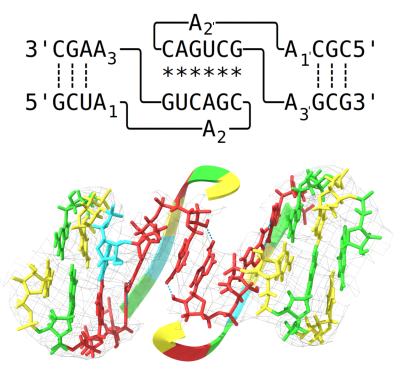
RNA therapeutics are emerging as a new way of targeting previously un-druggable therapeutic targets, and their full potential has yet to be realized. The focus thus far has been on RNA sequence, whereas next generation therapies will also utilize RNA structure. Current mRNA therapy design has optimized 5’ and 3’ untranslated regions (UTRS) as well as the coding sequence to maximize the translational output of mRNA. The 5’ and 3’ UTR sequences have been borrowed from naturally occurring sequences that have abundant translational output, resulting in an mRNA therapeutic that is ubiquitously expressed wherever the mRNA encounters translational machinery. However, key property of 5’ and 3’ UTRs is their complex 3D structure that allows them to regulate protein expression in a tightly controlled manner. Harnessing the full regulatory power of 5’ and 3’ UTRs for translational control of mRNA therapeutics would allow for expression of the mRNA payload only in the correct cellular context and reduce off-target effects. Achieving this regulatory specificity requires an understanding of RNA structural biology and the molecular interactions that govern the structure of 5’ and 3’ UTRs. Our research group uses cryo-EM structure determination and the design principles from the RNA origami method to modify RNA structures to improve the currently used 5’ and 3’ UTRs of mRNA therapies. By exploiting the cancer cell environment to enable cell state-specific expression and stabilization of the mRNA we hope to improve the efficacy of mRNA therapies for targeted neoadjuvant cancer treatments
